Iron-doped Nickel Oxide Nanoparticles Synthesis and Analyzing Different Properties
Volume 6, Issue 1, Page No 1422–1426, 2021
Adv. Sci. Technol. Eng. Syst. J. 6(1), 1422–1426 (2021);
 DOI: 10.25046/aj0601161
DOI: 10.25046/aj0601161
Keywords: Fe doped NiO, Optical studies, Nickel oxide nanoparticles, Optica bandgap, Morphological analysis
This is a report describing the impact of calcination on the morphological and optical properties of nanoparticles Iron doped-nickel oxide. By synthetic precipitation, the technique makes use of it. Three samples have been calcined in different temperatures and were characterized by X-ray diffraction (XRD), Fourier transforms infrared spectroscopy (FTIR), UV spectroscopy, and scanning electron microscopy (SEM), energy dispersive X-Ray (EDX). The study showed that the increase in the temperature enhances the structural and optical properties of the samples, and makes the samples take a more crystalline structure. It is also shown that there has been an expansion of the volume of the samples, making the samples having a small bandgap. UV-Visible absorption spectra of Iron doped-nickel oxide nitrate shows a peak of absorption between 350 to 400 nm. The bandgap value is calculated to be 1.86 eV at a calcination temperature of 350oC. The required structural and optical properties of Iron doped-nickel oxide nanoparticles make it a promising material for optoelectronic applications.
1.Introduction
There have been extensive studies about nanomaterials from the very beginning of the invention of it, due to their unique physical properties and bulk counterpart. Many methods are used to synthesize Nano-scale materials [1]. Oxidized metals are commonly used in numerous areas, such as photoelectric, sensors, recording materials, and catalysts, etc [2]. These applications can be enhanced and well-controlled by fabricating them into nano-sized materials. Nano-materials properties are peculiar and fascinating therefore scientists have been studying them intensely for the past few years. Among various nanomaterials, metal oxide as NiO has attracted technological and industrial interest for its optical-electronic, magnetic, thermal, and mechanical properties this metal is a good P-type semiconductor because of its wide bandgap [3]. Also, the high activity in the degradation of phenol and phenolic derivatives. There are several ways to synthesize it, such as sol-gel [4-7] co-precipitation [8] hydrothermal [9] solvothermal [10], and chemical precipitation [11, 12]. In this study, we prepared iron-doped NiO NPs using the chemical precipitation method. Previous studies showed the advantages of it, such as the simplicity and condition of the process, condition, crystal structure, and particle size can be more controllable. NiO is a transition metal oxide with a density of 6.67gm/cm3 and a melting point reaching 1955°C along with a self-ignition temperature of 400°C [13], nanoscale NiO particles are between 10 -30 nanometers and the surface area of NiO is about 130-150.
The XRD pattern of NiO NPs exhibits a face-centered cubic (FCC) crystalline structure [10]. The UV-IR spectrum of biosynthesized NiO Nanoparticles showed optical properties well-defined at 321 nm and its exhibited optical band gap is (3.83-3.18eV) [13- 16]. Furthermore, in previous studies, nanoparticles of NiO with different concentrations and calcination temperatures of Iron have been investigated, and it’s found that the properties of NiO NPs enhance with the addition of Fe and give excellent optical, structural, and magnetic properties. Therefore, this material can be a promising material when used in different applications such as optoelectronics, sensors, and batteries due its desired structural, optical, and magnetic properties. [8, 17, 18, 19, 20, 21, 22].
2. Experimental
2.1. Materials
The materials used in this research are: Nickel Nitride Ni (NO3)2, Iron nitride ( Fe2N ), Citric Acid (C6H8O7), and Ammonia hydrate (H6NO).Deionized water was used in all the experimental procedures.
2.2. Preparation of Iron doped NiO nanoparticles
In this research, NPs of Iron doped NiO samples have been prepared by dissolving 13.81 g of Nickel Nitrate Ni (NO3)2 and 1.01 g Iron nitrate Fe (NO3)3 into an aqueous solution of 50 ml distilled water under medium stirring speed and temperature of 50 C°. After 15 min, 1g of citric acid was added to the solution and kept stirring for another 15 minutes. Hereafter, Ammonium Hydrate (Liquid Ammonia) was added drop by drop, and precipitate started to appear until the mixture turned into a thick gel. Eventually, the temperature was raised to obtain a completely dry sample, which resulted in 5% Fe doped NiO nanoparticles. The dried samples were ground into a powder. In the end, the samples were placed in a muffle furnace at different temperatures ranging at (300,350,420) °C for 2 hours.
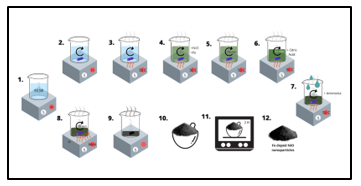 Figure 1: Iron-NiO Nanoparticles preparation method
Figure 1: Iron-NiO Nanoparticles preparation method
2.3. Measurements of microstructure and performance
This work aims to study the impact of calcination temperatures at 300, 350, and 420 oC on the structural and optical properties of Iron doped NiO samples. The structural properties of purity and crystalline Fe doped NiO were characterized by XRD measurements using Philips diffractometer with an accelerating voltage of (λ=1.54oA) with CuKα radiation. The morphological studies done by using a scanning electron microscope (SEM) have been carried out. equipped with EDAX to record the composition analysis (JEOL Model with an accelerating voltage of 30kV). The optical properties of the samples were calculated with the SHIMADZU double beam UV-visible spectrophotometer. Finally, Thermal analysis was carried out by Perkin-Elmer Fourier transform-infrared spectroscopy (FTIR) over the range of 590-4000cm-1.
3. Result
3.1. XRD Analysis
The structures of Iron-doped NiO nanoparticles calcined at 300, 350, 420 oC were analyzed using XRD and are represented in Fig.2. peaks of diffraction have been found near to 37.3°, 43.1°, 62.90°, 75.2°, and 79.3° in nanoparticles. However, both rhombohedral and cubic structural nanoparticles have been reported in reference [10, 17, 23]. Well-defined diffraction peaks for (111), (200), (311), and (222) cubic Iron-doped NiO NPs [10,17,22] were observed, which correspond to the standard spectrum (JCPDS, No.73-1519). At higher calcification temperatures, formed crystals get larger, which can be ascribed to thermally improved growth of crystals. The sizes of Iron doped NiO nanoparticles obtained from the diffraction ridges using the Scherrer equation are 5.6 nm and 9.6 nm and 11.6 nm for the sample calcined at 300oC, 350oC, and 420oC respectively, the sizes of nanoparticles increase with an increase in calcification temperatures and, the crystalline volume increases as the calcification temperature increases. W-H analysis was performed to calculate the volume and fine diffraction contributions for the XRD line expansion. The very small diffraction values of all samples result in a close agreement between the estimated crystalline sizes from Scherrer’s equation and the W-H analysis. The occurrence of vacancies and defects in composition Iron doped NiO nanoparticles can lead to subtle diffraction that leads to the widening of XRD peaks. From the Debye-Scherer equation, the average diameter of spherical particles can be easily calculated from
![]() where D represents the crystallite size, and K= 0.89 is the Scherrer constant and its associated with spherical particles and index (hkl) of the crystals. λ=1.54oA wavelength of source, β means the full width at half maximum (FWHM) of the peaks in radians. The average crystalline size increases from 4.83 nm to 14.5 nm as the calcination temperature increases and that is near previous studies [22,24].
where D represents the crystallite size, and K= 0.89 is the Scherrer constant and its associated with spherical particles and index (hkl) of the crystals. λ=1.54oA wavelength of source, β means the full width at half maximum (FWHM) of the peaks in radians. The average crystalline size increases from 4.83 nm to 14.5 nm as the calcination temperature increases and that is near previous studies [22,24].
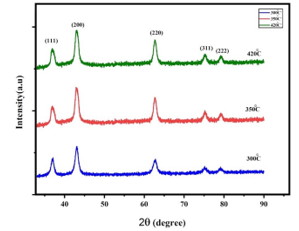 Figure 2: XRD structures of Iron doped NiO NPs samples at different calcination
Figure 2: XRD structures of Iron doped NiO NPs samples at different calcination
Temperatures
3.2. SEM Analysis and EDX
The particle size, shape, and morphology are identified using SEM micrographs. SEM images of the obtained Iron doped NiO NPs for the samples are shown in Fig.3. The SEM images show the morphology of the Iron doped NiO nanoparticles. It is observed that the shape of the particles is nearly spherical and Oval with non-homogeneous distribution, and they are in the range of 10 nm to 30 nm and that is close to the previous studies [15, 16, 24,4]. Particle size came out to be 4.6, 8.3, and 12.2 nm which means that particle size increases with the increase of the temperature. The particle size calculated from the SEM analysis seems to be quite similar to that measured from the XRD analysis of crystallite size. The EDX spectrum of Iron doped NiO nanoparticles is shown in Fig4. revealed the presence of Nickel, Oxygen, Carbon, and Iron is the only elementary species in samples.
3.3. FTIR Analysis
Figure 5 shows the FTIR spectra of Iron doped NiO particles samples calcined at 300, 350, and 420 oC. To determine the chemical structure of the samples the spectra were observed over the frequency range of 590-4000 cm-1. The broad absorption band centered at 3450 cm-1 is assigned to O–H stretching vibrations and the band at 1630 cm-1 is attributable to H–O–H bending vibration of water absorbed from the atmosphere when samples were prepared in the open air. Also, the absorption band in the region of 462–560 cm-1 is assigned to Fe–O stretching vibration mode and the second broad absorption band in the region 410 – 474 cm -1 is assigned to Ni–O stretching vibration mode. The broadness of the absorption band indicates that the NiO powders are nanocrystals and well crystallized. The intensity of Ni – O and F – O stretching bands seen in Fig.5 are increasing as the calcination.
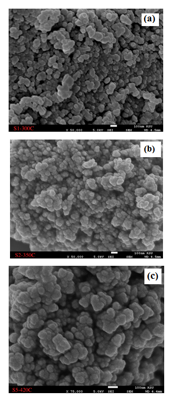 Figure 3: SEM images of Iron doped NiO NPs Calcined at (a)300, (b) 350, and (c) 420 °C.
Figure 3: SEM images of Iron doped NiO NPs Calcined at (a)300, (b) 350, and (c) 420 °C.
3.4. UV-Visible studies
Figure 6 shows spectra for UV-visible absorption and (αhν)2 versus energy plot for Iron doped NiO nanoparticles samples. UV studies of absorption showed that a rise in calcination temperature results in a redshift in the absorption spectrum and a decrease in bandgap as a result of an increase in particle size. The peaks range in the ultraviolet spectrum showed between (300-400 nm) and that is close to the previous studies [10, 15]. In general, the data show that the sample calcined at 300oC has a value of 307 nm, and the value of 347 nm for the sample calcined at 350oC, lastly the value 436 nm for the sample calcined at 420oC, the peaks mean the matter absorption of the spectrum and also work to stimulate the electrons. The best result of the Three samples is the sample calcined at 350oC with a bandgap value of 1.86 eV and high absorption value which makes the bandgap a small value. At calcination temperatures, the properties of the material are enhanced.
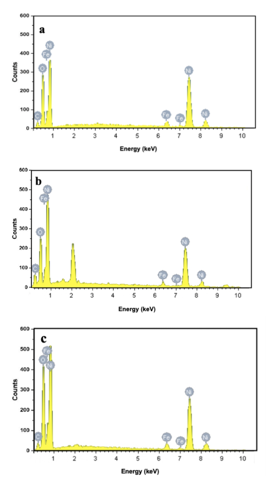 Figure 4: EDX spectra of Iron doped NiO NPs calcined at (a)300°C (b) 350°C, and (c) 420°C.
Figure 4: EDX spectra of Iron doped NiO NPs calcined at (a)300°C (b) 350°C, and (c) 420°C.
4. Conclusions
Nanostructured Iron doped NiO particles were prepared successfully utilizing nickel nitrate hexahydrate and ammonium carbonate via the chemical precipitation method. The results obtained from XRD is that the crystalline structure of nanoparticles has been improved due to the addition of Fe and calcined samples at different temperatures to be FCC, this observation is consistent with the previous studies. Furthermore, UV-visible absorption results have shown that a rise in the temperature of calcination causes a redshift in the absorption spectrum and a decrease in the bandgap due to an increase in the size of the particles. We can say calcination samples at high temperatures with a constant concentration of Fe are the main parameters of the appearance of a redshift in our results. Also, the particle size decreases with an increase in the dopant concentration of Fe. Based on these systematic observations, it is concluded that calcined NiO doped with 5% Fe nanoparticles may have been an outstanding option for optoelectronic applications because of its desired structural and optical properties.
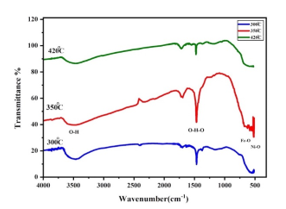 Figure 5: illustrates the FTIR spectra of the samples
Figure 5: illustrates the FTIR spectra of the samples
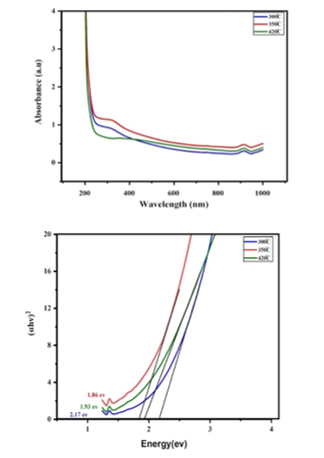 Figure.6 a- UV-Visible absorbance spectra. b-(αhν)2 versus energy plot for NiO3+FeO3 nanoparticles.
Figure.6 a- UV-Visible absorbance spectra. b-(αhν)2 versus energy plot for NiO3+FeO3 nanoparticles.
5. Acknowledgments
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, Riyadh, Kingdom of Saudi Arabia. In addition, we would like to remember Maha Almoneef, Fatima Alkalas, and Nourah Alwadei at the Department of Physics, College of Science at Princess Nourah Bint Abdulrahman University, for their contributions in this article
- H. Gleiter, “Nanocrystalline Materials,” in: Bunk, W. G. J., ed., in Advanced Structural and Functional Materials, Springer Berlin Heidelberg, Berlin, Heidelberg: 1–37, 1991.
- J. Shi, X. Zhang, W. Baeyens, A.M. García-Campaña, “Recent developments in nanomaterial optical sensors,” TrAC Trends in Analytical Chemistry, 23, 351–360, 2004, doi:10.1016/S0165-9936(04)00519-9.
- R. Palombari, “Influence of surface acceptor–donor couples on conductivity and other electrochemical properties of nonstoichiometric NiO at 200°C,” Journal of Electroanalytical Chemistry, 546, 23–28, 2003, doi:https://doi.org/10.1016/S0022-0728(03)00134-7.
- N.N.M. Zorkipli, N.H.M. Kaus, A.A. Mohamad, “Synthesis of NiO Nanoparticles through Sol-gel Method,” Procedia Chemistry, 19, 626–631, 2016, doi:https://doi.org/10.1016/j.proche.2016.03.062.
- P. Mallick, C. ~S. Sahoo, N. ~C. Mishra, “Structural and optical characterization of NiO nanoparticles synthesized by sol-gel route,” in: Bose, S. ~M. and Tripathy, S. ~K., eds., in Functional Materials, 229–232, 2012, doi:10.1063/1.4736893.
- P. Jeevanandam, V.R.R. Pulimi, “Synthesis of nanocrystalline NiO by sol-gel and homogeneous precipitation methods,” Indian Journal of Chemistry – Section A Inorganic, Physical, Theoretical and Analytical Chemistry, 51, 586–590, 2012.
- M. Alagiri, S. Ponnusamy, C. Muthamizhchelvan, “Synthesis and characterization of NiO nanoparticles by sol–gel method,” Journal of Materials Science-Materials in Electronics – J MATER SCI-MATER ELECTRON, 23, 2012, doi:10.1007/s10854-011-0479-6.
- P. Mallick, C. Rath, R. Biswal, N.C. Mishra, “Structural and magnetic properties of Fe doped NiO,” Indian Journal of Physics, 83, 517–523, 2009, doi:10.1007/s12648-009-0012-4.
- S. Takami, R. Hayakawa, Y. Wakayama, T. Chikyow, “Continuous hydrothermal synthesis of nickel oxide nanoplates and their use as nanoinks for p-type channel material in a bottom-gate field-effect transistor,” Nanotechnology, 21, 134009, 2010, doi:10.1088/0957-4484/21/13/134009.
- W. Duan, S. Lu, Z. Wu, Y. Wang, “Size Effects on Properties of NiO Nanoparticles Grown in Alkalisalts,” The Journal of Physical Chemistry C, 116, 26043–26051, 2012, doi:10.1021/jp308073c.
- V. Biju, M. Khadar, “Analysis of AC Electrical Properties of Nanocrystalline Nickel Oxide,” Materials Science and Engineering: A, 304, 814–817, 2001, doi:10.1016/S0921-5093(00)01581-1.
- S. Chakrabarty, K. Chatterjee, “Synthesis and Characterization of Nano-Dimensional Nickelous Oxide (NiO) Semiconductor,” J. Phys. Sci., 13, 2009.
- M.A. Marselin, N. Jaya, “Synthesis and characterization of Pure and Cobalt-doped NiO Nanoparticles,” 2015.
- M. rifaya, T. Thirugnanasambandan, M. Alagar, “Chemical Capping Synthesis of Nickel Oxide Nanoparticles and their Characterizations Studies,” Nanoscience and Nanotechnology, 2, 2012, doi:10.5923/j.nn.20120205.01.
- P. Sheena, P. K P, A. Nedumkallel, B. Sabu, T. Varghese, M. Asmabi, “EFFECT OF CALCINATION TEMPERATURE ON THE STRUCTURAL AND OPTICAL PROPERTIES OF NICKEL OXIDE NANOPARTICLES,” 5, 2014.
- K.N. Patel, M.P. Deshpande, V.P. Gujarati, S. Pandya, V. Sathe, S.H. Chaki, “Structural and optical analysis of Fe doped NiO nanoparticles synthesized by chemical precipitation route,” Materials Research Bulletin, 106, 187–196, 2018, doi:10.1016/j.materresbull.2018.06.003.
- P.M. Ponnusamy, S. Agilan, N. Muthukumarasamy, T.S. Senthil, G. Rajesh, M.R. Venkatraman, D. Velauthapillai, “Structural, optical and magnetic properties of undoped NiO and Fe-doped NiO nanoparticles synthesized by wet-chemical process,” Materials Characterization, 114(C), 166–171, 2016, doi:10.1016/j.matchar.2016.02.020.
- K. R, J. G, A.A. Alphonse, A. Raj, “Structural and Magnetic Properties of NiO and Fe-doped NiO Nanoparticles Synthesized by Chemical Co-precipitation Method,” Materials Today: Proceedings, 3, 1370–1377, 2016, doi:10.1016/j.matpr.2016.04.017.
- H. Abbas, K. Nadeem, A. Hassan, S. Rahman, H. Krenn, “Enhanced photocatalytic Activity of Ferromagnetic Fe-doped NiO nanoparticles,” Optik, 202, 163637, 2020, doi:https://doi.org/10.1016/j.ijleo.2019.163637.
- R. Pradeep, A.C. Gandhi, Y. Tejabhiram, I.K.M.M. Sahib, Y. Shimura, L. Karmakar, D. Das, S.Y. Wu, Y. Hayakawa, “Magnetic anomalies in Fe-doped {NiO} nanoparticle,” Materials Research Express, 4(9), 96103, 2017, doi:10.1088/2053-1591/aa7f9b.
- H. Muthusamy, N. Suriyanarayanan, S. Prabahar, “Nanoscale synthesis and optical features of nickel nanoparticles,” Optik – International Journal for Light and Electron Optics, 125, 1962–1966, 2014, doi:10.1016/j.ijleo.2013.09.069.
- K. Krishnan, K. Selvan, M. Kanagaraj, S. Arumugam, N. Jaya, “Particle size effect on the magnetic properties of NiO nanoparticles prepared by a precipitation method,” Journal of Alloys and Compounds – J ALLOYS COMPOUNDS, 509, 181–184, 2011, doi:10.1016/j.jallcom.2010.09.033.
- Z.M. Khoshhesab, M. Sarfaraz, “Preparation and Characterization of NiO Nanoparticles by Chemical Precipitation Method,” Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 40(9), 700–703, 2010, doi:10.1080/15533174.2010.509710.
- M. Bonomo, “Synthesis and characterization of NiO nanostructures: a review,” Journal of Nanoparticle Research, 20, 2018, doi:10.1007/s11051-018-4327-y.
No related articles were found.
