Evaluation of Physicochemical Stability in Extemporaneous Omeprazole-Based Preparations
Volume 10, Issue 1, Page No 7–12, 2025
Adv. Sci. Technol. Eng. Syst. J. 10(1), 7–12 (2025);
 DOI: 10.25046/aj100102
DOI: 10.25046/aj100102
Keywords: Omeprazole, Stability, Extemporaneous preparation
Extemporaneous omeprazole-based preparations are commonly used in hospitals; however, there are no validated studies about physicochemical stability. This study aimed to determine if temperature, luminosity, and the type of diluent affect the stability of omeprazole in the extemporaneous preparation. For stability, the methodology validated previously by our group was used. The 2k experimental design included Temperature (25ºC and 35ºC) and Luminosity (covered by light and 400 Lx) variables. Diluents were evaluated at five levels: 1) Citric acid + polyethylene glycol solution, 2) Polyethylene glycol solution, 3) Physiological saline solution, 4) Citric acid + polyethylene glycol solution + physiological saline solution, and 5) Polyethylene glycol solution + physiological saline solution. Minitab 18 software was used for data analysis, and the degradation kinetics were determined by linear regression. The optimal condition for physicochemical stability was a temperature of 25ºC covered by light (OM1-3h 49’, OM2-3h 2’, SSF-5h 8’, OM1SSF-2h 27’, OM2SSF-6h 23’). The diluent based on physiological saline solution provided more than five hours of shelf-life, and more than six hours, the diluent based on OM2 + SSF. However, the best option is physiological saline solution, considering the accessibility of the diluent. In conclusion, environmental conditions should be considered in extemporaneous omeprazole-based preparation since they are affected by temperature, luminosity, and type of diluent. Assessing shelf-life prior to administration is necessary to provide a safe and effective drug, avoiding the occurrence of side effects in patients.
1. Introduction
Omeprazole (OMZ) is one of the most widely used drugs in hospitals, increasing its use every year. However, the indiscriminate use of extemporaneous omeprazole-based preparations represents a health risk. Up to 73% of patients who receive omeprazole do not require it, and 38% of these have shown adverse effects, which can prolong their hospital stay [1], [2].
The requirements for evaluating stability are indicated in the NOM-073 “Stability of drugs and medicines, as well as herbal remedies” for determining the shelf-life of medicines as marketed by the pharmaceutical industry [3]. However, in the case of extemporaneous drugs, there is no national regulation; the shelf-life should be determined by the conditions in which the extemporaneous preparation is made. Proper preservation is a prerequisite to maintaining the pharmacological and therapeutic properties. Therefore, it is important to improve safety since it can decrease effectiveness and modify safety due to the toxicity of degradation products [4].
Moreover, information on the stability and storage conditions of extemporaneous preparations is limited since pharmacovigilance is not a common practice in our country. In addition, generally, the unit in charge of performing such activity is the nursing. This can lead to errors, such as inadequate formulation, microbial contamination, concentration, and wrong dose calculation. Therefore, it is essential to evaluate the stability of the drug prior to its administration to patients to avoid the risk of adverse effects or toxicity [5].
The main conditions to retain the physicochemical stability and the microbiological properties within the quality specifications established in the formulation during its shelf-life and throughout storage time, are temperature, humidity, luminosity, and type of diluent [4], [6], [7]. Such variables can accelerate the degradation kinetics of active ingredients and alter their efficacy and safety [2], [3].
The shelf-life of a drug is measured by the concentration of the active ingredient, which should be < 10% of the total dose indicated on the product label. A percentage greater than 10% pointed out that the drug is losing effectiveness. Also, the degradation products could cause adverse or toxic effects on the patients [3], [5], [8], [9], [10], [11].
The 2k design systematizes and reduces the variables to simplify the procedure and evaluates the main effects and their interactions [12]. In 2k factorial designs, the 2 represents the two levels at which each variable is tested, identified as low (-) and high (+). The k represents each of the independent variables. The design (2*2) performs four different experiments; the variables are identified with the letters A and B, and the interaction between these two variables is identified with AB [13], [14].
Additionally, photosensitive drugs need to be kept covered by light due to their characteristics [11]. Those indications are usually mentioned in the package insert or technical data sheet. All photosensitive drugs should be kept in appropriate containers (protected from light) to avoid deterioration, both in the pharmaceutical services and the hospitalization units. Many of them are packaged by the pharmaceutical industry (in topaz glass ampoules) to protect them from light. If this does not occur, they should always be kept in the original package or wrapped in aluminum foil or other opaque paper [11].
In the internal medicine area of a tertiary care hospital in the state of Veracruz, a medication preparation service has been available since 2014, which has allowed a decrease in the adverse effects associated with medication (dosage, preparation, among others). However, there are no stability studies on extemporaneous preparations. Therefore, this study aimed to determine whether the factors of temperature, luminosity, and type of diluent are associated with the stability of omeprazole in extemporaneous preparations.
2. Methods
An experimental study was carried out in the Pharmaceutical Technology Laboratory of the School of Pharmaceutical Biological Chemistry, Universidad Veracruzana, Mexico.
The experimental steps carried out in this study are described in Figure 1.
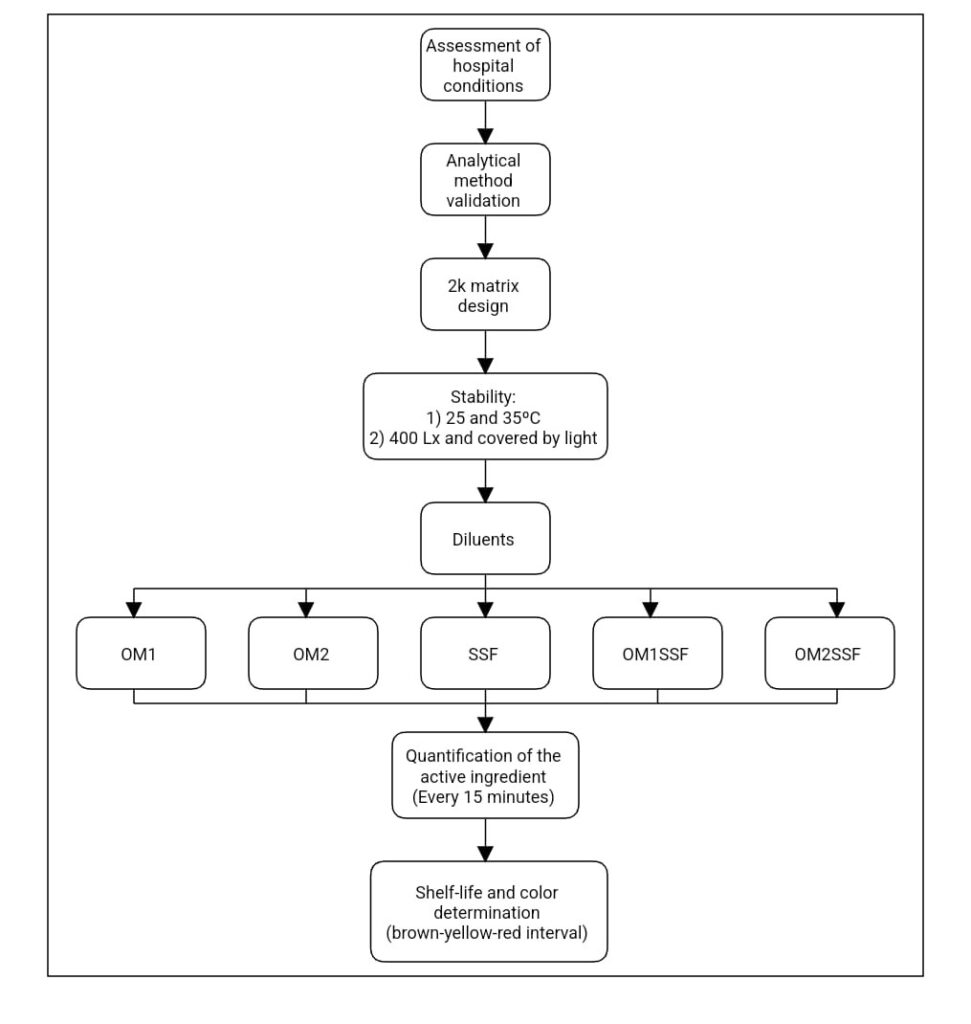
2.1. Reagents:
The hospital provided two intravenous omeprazole trademarks (OMZ1 lot T18J487 and OMZ2 lot OM18I15). The OMZ reference standard was purchased from Sigma-Aldrich, purity 99.6%, lot LRAC0716, with the supplier’s certificate of analysis.
2.2. Analytical equipment:
– UV Spectrophotometer, Beckman, model DU-7000.
– Stove, RIOS Rocha, model HS-33 adapted with 400Lx LED lamp.
2.3. Matrix of 2k desing:
In this study a factorial design was used, since in each trial or complete replication of the experiment all possible combinations of factor levels were investigated [15]. Factorial designs produce more adequate experiments, since each observation provides information on all factors. Thus, the response to any factor observed under different conditions indicates whether the factors act independently on the experimental units. Interaction between factors occurs when their action is not independent [16].
The independent variables evaluated were Temperature, Luminosity, and Type of diluent. The dependent variable was the shelf-life. We performed a 2k design to determine the association of factors involved in the stability of the active agent [13], [14]. Analyses were repeated ten times until the concentration of the active in the extemporaneous preparation decreased by more than 10 percent. Temperature includes 25 and 35 degrees Celsius (ºC). Luminosity includes covered by light and 400 Luxes (Lx). The diluent was evaluated at five levels, described in the next section. The variables and their levels are shown in Table 1.
The conditions used in this study were according to standards. The temperature of 25ºC is the optimal for the preparation and storage of non-thermolabile drugs. The second temperature was selected after reviewing the areas of administration of the extemporaneous preparations, with the maximum temperature at 35ºC. Concerning the luminosity, 400 Lx is what a standard hospital lamp provides.
Table 1. Factors and levels included in the 2k desing.
| Factors | Levels | |
| Low (-) | High (+) | |
| Temperature | 25ºC | 35ºC |
| Luminosity | Covered by light | 400 Lx |
Lx= luxes.
The proposed design (2k) defines a total of 4 tests for each evaluation. The combinations used in each test are presented in Table 2.
Table 2. Matrix of 2k desing
| Test | Temperature | Luminosity |
| 1 | (-) 25ºC | (-) Covered by light |
| 2 | (+) 35ºC | (-) Covered by light |
| 3 | (-) 25ºC | (+) 400 Lx |
| 4 | (+) 35ºC | (+) 400 Lx |
Lx= luxes.
For each diluent, a four-test assay matrix was assigned. The diluent preparation is shown below:
Diluents:
-SSF: Intravenous OMZ diluted with Physiological Saline Solution 0.9%.
-OM1: Intravenous OMZ diluted with its diluent (Solution with Citric Acid + Polyethylene Glycol).
-OM2: Intravenous OMZ diluted with its diluent (Polyethylene glycol solution).
-OM1SSF: Intravenous OMZ diluted with OM1 + SSF (50:50).
-OM2SSF: Intravenous OMZ diluted with OM2 + SSF (50:50).
SSF, OM1, and OM2 are commercial diluents that the hospital provides and used in the internal medicine area. The manufacturers did not indicate the OM1 and OM2 diluent concentrations. A matrix of two hundred determinations was performed considering the design matrix (4 tests), the diluents (5), and the ten sampling times.
Quantification method (Validation and quantification):
The OMZ quantification was previously reported and validated by our working group [17].
Stability:
Physicochemical stability (quantification of OMZ) was performed at the conditions of 25°C ± 2°C and 35°C ± 2°C, with a lamp providing 400 Lx. Samples prepared with the diluents were analyzed each 15 min until the concentration of OMZ decreased > 10% [3].
Color determination
The method was obtained from the analysis 0181 “Solution color” described in the United Mexican States Pharmacopoeia (2014). It is based on the visual color of the sample (in solution) against reference standards in a specific color range under established conditions [18].
The color presented in the sample, will be within the brown-yellow-red interval. A solution is considered colorless if its appearance is the same as that of the water or solvent used to reconstitute it (not more intense than the reference solution B9)
Preparation of standard solutions:
Solutions were prepared as indicated in Annex II of the FEUM “Preparation of reference solutions.”
Procedure:
We prepared reference solutions in 10 mL tubes of equal diameters. Then, we transfer 5 mL of the sample of omeprazole preparation to a 10 mL test tube of equal diameter to those of the reference solutions. Compare the sample with the reference solutions in a horizontal plane separated from each other by 3 cm on a white background with indirect light.
1. Results and discussion
Shelf-life
The results of the stability of the drug OMZ in its different extemporaneous preparations are shown in Table 3 and Figure 2, where the shelf-life times (time in hours to reach 10% degradation of the active principle) are presented.
Regarding shelf-life, the optimal condition is covered by light at 25°C (Test 1). According to the literature, this condition prevents the degradation of the active ingredients [19], [20].
The diluents with the best shelf-life times (obtained in all the conditions evaluated) were SSF and OM2SSF (Table 3 and Figure 2).
Table 3. Shelf-life times (hours).
| Tests | Diluents | ||||
| OM1 | OM2 | SSF | OM1SSF | OM2SSF | |
| 1 | 3h 49’ | 3h 2’ | 5h 8’ | 2h 27’ | 6h 23’ |
| 2 | 01h 4’ | 01h 16’ | 02h 25’ | 01h 10’ | 01h 22’ |
| 3 | 01h 57’ | 01h 57’ | 01h 48’ | 01h 48’ | 02h 20’ |
| 4 | 01h 4’ | 01h 15’ | 02h 19’ | 02h 00’ | 01h 6’ |
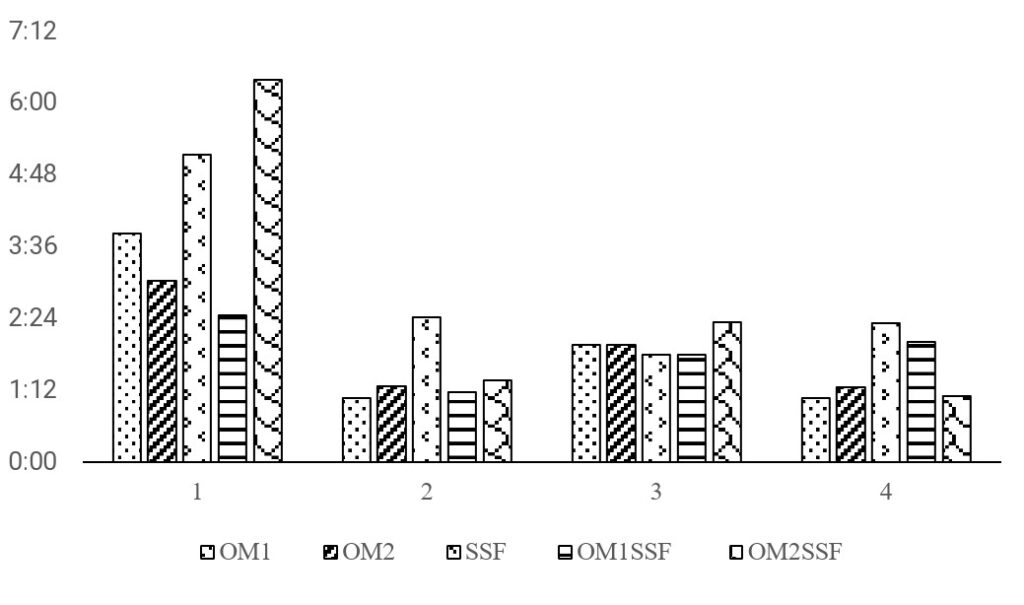
The above results differ from those reported in the Stabilis 4.0 page, which indicates shelf-life times of 6 hours with 5% glucose solution covered by light, without considering the temperature. The values closest to those previously reported are those obtained for preparations with OM2SSF diluent (06h 23), followed by the preparation with SSF diluent (05h 08) of shelf-life, both at conditions of 25°C and covered by light. The literature indicates that preparations with the SSF-based diluent presents a shelf-life of 12 hours. However, the present study shows lower shelf-life times than previously reported. Those results allow us to point out that it is necessary to verify the shelf-life times reported in the literature and check the operating conditions of each institution center.
In the tertiary hospital where extemporaneous preparations of OMZ are prepared, the shelf-life used is four hours, according to the information indicated in the inserts (at room temperature <no more than 30°C> covered by light).
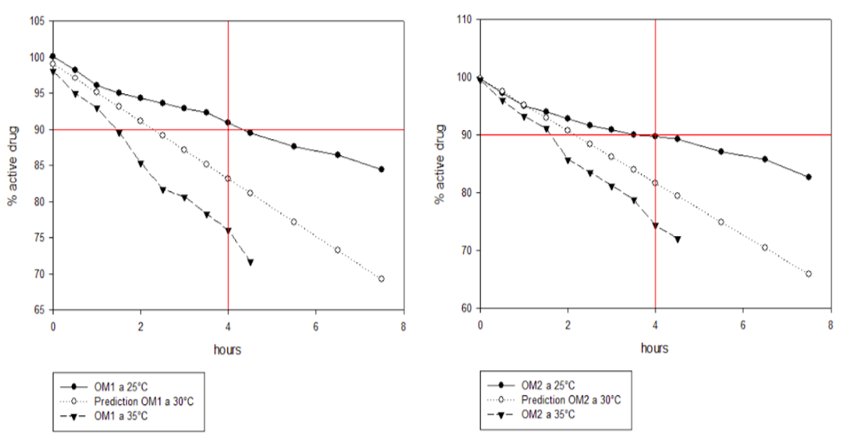
Concerning the results obtained in this study, the stability conditions recommended by the manufacturers in the insert were not met. For OM1 (diluent with citric acid and polyethylene glycol), under the conditions of 25°C and covered by light, a shelf-life of 03h 49 hours was obtained. For the OM2 solution (diluent with polyethylene glycol), a shelf-life of 03h 02 hours was obtained. On the other hand, the conditions of 35°C and 400 Lx (OM1 and OM2) showed a shelf-life above two hours for both solutions without completing the shelf-life mentioned in the insert.
Figure 3 shows the predictions obtained using the Arrhenius method for the 30°C condition of the diluents of solutions OM1 and OM2, where the comparison with the temperatures of 25°C (circle) and 35°C (triangle), both covered by light, can be seen. The red line on the Y axis indicates 90% of the active; the X axis indicates the time marked on the label of the two drugs (4 hours).
Analysis of main effects (2k design)
The original design proposed in this study is a 2k, which considers the variables temperature and luminosity for each diluent analyzed. The results obtained for the main effects and interaction are reported below.
Regarding the analysis of the main effects (Figure 4), the impact of temperature on the shelf-life is more significant in the average variation of response compared to luminosity in the different tests with the diluents, except the SSF where the main effect is luminosity compared to temperature. The plots of the main effects corroborate the significance of each of the diluents tested.
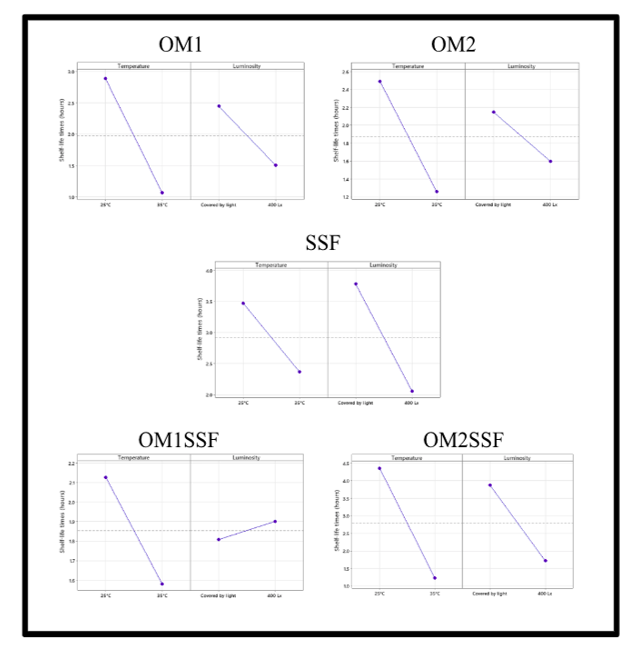
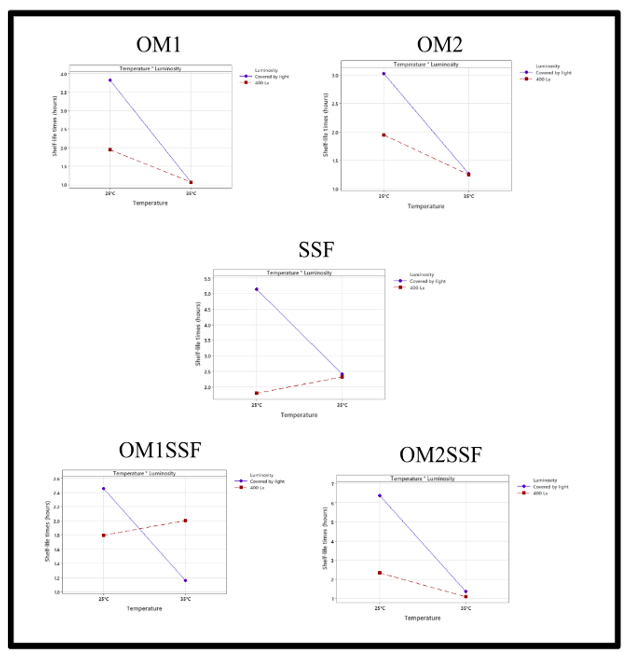
The SSF diluent is the best option for the extemporaneous preparation of OMZ, considering the luminosity, since it is the variable that most affects it. Those will provide better control of the preparation since it is easier to control the luminosity [11].
The diluent least affected by the main effects and by the interaction is the OM2SSF, presenting good shelf-life times. The difficulty of the preparation is the availability of the diluent polyethylene-glycol since its preparation is limited to the manufacturer’s availability [7].
On the other hand, the diluent with the greatest variation was the OM1SSF, where temperature and luminosity decreased the shelf-life. This preparation presented the biggest interaction and the lowest shelf-life.
Solution color
Simultaneously to the quantification of the active ingredient, color determinations were made by comparison with standards. The results are presented in Figure 4.

Omeprazole is a photosensitive drug, so when exposed to light, it shows slight coloration, as evidenced in this test. Tables 4, 5, and 6 show the samples that presented coloration when exposed to 400 Lx. The coloration with the greater intensity was at 25ºC temperature. There were no visual differences between the diluents (OM1 versus OM2, and SSF versus OM2SSF) (Tables 4 and 5) except the OM1SSF mixture (Table 6), which exhibits a coloration that occurs at 25ºC with 400 Lx.
In this analysis, there is no direct relationship between the appearance of color in the preparation and the degradation of omeprazole. However, there is a direct relationship between the color appearance in the solution and the exposure to 400 Lx luminosity. These results suggested a decrease in the appearance of color in extemporaneous preparations of omeprazole intravenous solution.
One of the main limitations of this study is that stability studies could not be performed during storage time; however, the results indicate that pharmacovigilance studies applied to extemporaneous preparations are necessary to inform the nursing department about the correct conditioning of the extemporaneous preparation to provide a safe and effective drug, avoiding the occurrence of side effects in patients [5].
Table 4. Staining of the diluent solution OM1 and OM2.
| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | ||
| 25°C – C/L | GY7 | GY7 | GY7 | GY7 | GY7 | GY6 | GY6 | GY5 | GY4 | GY3 | |
| 35°C – C/L | GY7 | GY7 | GY7 | GY6 | GY6 | GY6 | GY5 | GY4 | GY4 | GY3 | |
| 25°C – 400lx | GY7 | GY7 | GY6 | GY6 | GY4 | GY3 | GY3 | GY2 | GY2 | GY2 | |
| 35°C – 400lx | GY7 | GY7 | GY7 | GY6 | GY5 | GY5 | GY4 | GY3 | GY2 | GY2 |
GY = color scale according to pharmacopoeia within the brown-yellow-red interval. GY1 represents the highest intensity. C/L = cover of light. No difference was observed between solutions OM1 and OM2.
Table 5. Staining of the diluent solution SSF and OM2SSF
| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | |
| 25°C – C/L | GY7 | GY7 | GY7 | GY7 | GY6 | GY6 | GY5 | GY5 | GY4 | GY3 |
| 35°C – C/L | GY7 | GY7 | GY7 | GY6 | GY6 | GY6 | GY5 | GY4 | GY4 | GY3 |
| 25°C – 400lx | GY7 | GY7 | GY6 | GY6 | GY4 | GY3 | GY3 | GY2 | GY2 | GY2 |
| 35°C – 400lx | GY7 | GY7 | GY7 | GY6 | GY5 | GY5 | GY4 | GY3 | GY2 | GY2 |
GY = color scale according to pharmacopoeia within the brown-yellow-red interval. GY1 represents the highest intensity. C/L = cover of light. No difference was observed between solutions SSF and OM2SSF.
Table 6. Staining of the diluent solution OM1SSF
| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | |
| 25°C – C/L | GY7 | GY7 | GY7 | GY7 | GY6 | GY6 | GY5 | GY4 | GY3 | GY3 |
| 35°C – C/L | GY7 | GY7 | GY7 | GY6 | GY6 | GY5 | GY4 | GY4 | GY3 | GY3 |
| 25°C – 400lx | GY7 | GY6 | GY6 | GY6 | GY3 | GY3 | GY2 | GY2 | GY2 | GY2 |
| 35°C – 400lx | GY7 | GY7 | GY7 | GY6 | GY5 | GY4 | GY4 | GY3 | GY2 | GY2 |
GY = color scale according to pharmacopoeia within the brown-yellow-red interval. GY1 represents the highest intensity. C/L = cover of light.
4. Conclusion
The stability of the extemporaneous preparation of OMZ -intravenous solution is affected by temperature, luminosity, and type of diluent. Temperature was the variable with the main impact on shelf-life, with the shelf-life at 25°C except for the SSF solution, whose effect was mainly due to luminosity. Regarding luminosity, the optimal condition is preparing extemporaneous solutions covered by light. The optimal diluent for the extemporaneous preparation of OMZ is based on a physiological saline solution due to its longer shelf-life and no commercial brand limitations for its preparation. Concerning the staining of solutions, it is necessary to protect Omeprazole preparations from light, regardless of the diluent used, to ensure the effectiveness and safety of the drug. These conditions are necessary for the good management of omeprazole in the hospital setting, for its suitability for clinical use, and to avoid ineffectiveness and toxic effects associated with its irrational use.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgment
The authors thank the Centro de Alta Especialidad “Dr. Rafael Lucio” for the support of this work.
- F. Carranza, “Seguridad del omeprazol: ¿es adecuada la duración de los tratamientos?” Farmacéuticos Comunitarios, 7(1), 5–9, 2015, https://doi.org/10.5672/fc.2173-9218.(2015/vol7).001.02.
- A. Castro, C. Martín De Argila, A. Albillos, “Consideraciones prácticas en el manejo de los inhibidores de la bomba de protones”, Revista Española de Enfermedades Digestivas (Madrid), 108, 145–153. 2016, http://scielo.isciii.es/pdf/diges/v108n3/es_revision.pdf
- Secretaría de Salud, “NOM-073-SSA1-2005, Estabilidad de fármacos y medicamentos”, 2005, https://salud.gob.mx/unidades/cdi/nom/073ssa105.html
- J.R. Falconer, K.J. Steadman, “Extemporaneously compounded medicines”, Australian Prescriber, 40(1), 5–8, 2017, https://doi.org/10.18773/austprescr.2017.001
- M.R. Mattos da Silva, D.L. Pereira, E. Pereira dos Santos, J.E. Ricci, “Preparation of extemporaneous oral liquid in the hospital pharmacy”. Brazilian Journal of Pharmaceutical Sciences, 56, e18358, 2020, https://doi.org/10.1590/s2175-97902019000418358
- V.K. Yellepeddi, “Stability of extemporaneously prepared preservative-free prochlorperazine nasal spray”, American Journal of Health-System Pharmacy, 75(1), e28–e35, 2018, https://doi.org/10.2146/ajhp160531
- N. Barrueco, I. Escobar-Rodríguez, B. García-Díaz, M.E. Gil-Alegre, E. López-Lunar, M.G. Ventura Valares, “Estabilidad de medicamentos en la práctica clínica: de la seguridad a la eficiencia”, Farmacia Hospitalaria, 37(3), 175-177, 2013, https://dx.doi.org/10.7399/FH.2013.37.3.587
- M. Yu, J. Qian, D. Guo, L. Li, X. Liu, “Severe adverse reactions caused by omeprazole: A case report”, Experimental and Therapeutic Medicine, 12(2), 1103–1106, 2016, https://doi.org/10.3892/etm.2016.344V
- FEUM, “Suplemento para establecimientos dedicados a la venta y suministro de medicamentos y demás insumos para la salud”, 6a. Edición, 2019, México.
- E. Casaus, “Guía de buenas prácticas en la administración de medicamentos en servicios de farmacia hospitalaria”, Farmacia Hospitalaria, 2024, https://www.sefh.es/sefhpdfs/GuiaBPP_JUNIO_2014_VF.pdf
- I. Sánchez, M.D. Nájera, A. Espuny, J.C. Titos, “Revisión de la estabilidad de los medicamentos fotosensibles”, Farmacia Hospitalaria, 35(4), 204–215, 2011, https://doi.org/10.1016/j.farma.2010.05.005
- A. Correa, P. Grima, X. Tort-Martorell, “Experimentation order with good properties for 2k factorial designs”, Journal of Applied Statistics, 36(7), 743–754, 2009, https://doi.org/10.1080/02664760802499337
- J. Lu, “On finite-population Bayesian inferences for 2k factorial designs with binary outcomes”, Journal of Statistical Computation and Simulation, 89(5), 927–945, 2019, https://doi.org/10.1080/00949655.2019.1574793
- P. Paengkoum, C. Yuangklang, S. Paengkoum, “Robust 2k factorial designs: non-normal symmetric distributions”, Pakistan Journal of Statistics, 77(2555), 73-77, 2012, https://avesis.anadolu.edu.tr/yayin/5e5440d7-fa53-4f62-b152-d7f40e35d981/robust-2k-factorial-designs-non-normal-symmetric-distributions
- D. Montgomery, “Diseño y análisis de experimentos”, 2a. Edición, Limusa Wiley; 2010, México.
- L. Kuehl, “Diseño de Experimentos: Principios estadísticos para el diseño y análisis de investigaciones”, 2a. Edición, Thomson Learning; 2001, España.
- E. Cruz, I. Camacho, J. Locia, L.I. Pascual, M.O. Pérez, J.J. Diaz, “Validation of a UV spectrophotometric method for quantification of Omeprazole using different types of diluents”, IEEE International Conference on Engineering Veracruz (ICEV), 2023, doi: 10.1109/ICEV59168.2023.10329709
- FEUM, “Métodos generales de análisis”, 11a. Edición, 2014, México.
- Secretaria de Salud, “NORMA Oficial Mexicana NOM-249-SSA1-2010, Mezclas estériles: nutricionales y medicamentosas, e instalaciones para su preparación”, 2010, http://www.dof.gob.mx/normasOficiales/4327/salud/salud.htm
- A. Tellez, “Modelo nacional de farmacia hospitalaria”, Secretaria de Salud, 2009, https://www.uaeh.edu.mx/investigacion/icsa/LI_UsoMedic/Ana_Tellez/modelo.pdf
- Falko Gawantka, Franz Just, Marina Savelyeva, Markus Wappler, Jörg Lässig, "A Novel Metric for Evaluating the Stability of XAI Explanations", Advances in Science, Technology and Engineering Systems Journal, vol. 9, no. 1, pp. 133–142, 2024. doi: 10.25046/aj090113
- Abdul Ahad Jhumka, Robert Tat Fung Ah King, Chandana Ramasawmy, Abdel Khoodaruth, "Proportional Derivative and Proportional Integral Derivative Controllers for Frequency Support of a Wind Turbine Generator in a Diesel Generation Mix", Advances in Science, Technology and Engineering Systems Journal, vol. 8, no. 4, pp. 60–65, 2023. doi: 10.25046/aj080407
- Abdul Ahad Jhumka, Robert Tat Fung Ah King, Chandana Ramasawmy, "Assessing the Impact of Integrating Solar PV System using the Equal Area Criterion Method", Advances in Science, Technology and Engineering Systems Journal, vol. 7, no. 5, pp. 35–40, 2022. doi: 10.25046/aj070505
- Christoph Rüeger, Jean Dobrowolski, Petr Korba, Felix Rafael Segundo Sevilla, "Analysis of Grid Events Influenced by Different Levels of Renewable Integration on Extra-large Power Systems", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 5, pp. 43–52, 2021. doi: 10.25046/aj060506
- Akintunde Alayande, Somefun A.O, Tobiloba Somefun, Ademola Ademola, Claudius Awosope, Obinna Okoyeigbo, Olawale Popoola, "Transient Stability Enhancement of a Power System Considering Integration of FACT Controllers Through Network Structural Characteristics Theory", Advances in Science, Technology and Engineering Systems Journal, vol. 6, no. 1, pp. 968–981, 2021. doi: 10.25046/aj0601107
- Nagaraj Vannal, Saroja V Siddamal, "Design and Implementation of DFT Technique to Verify LBIST at RTL Level", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 6, pp. 937–943, 2020. doi: 10.25046/aj0506111
- Marco Bindi, Igor Aizenberg, Riccardo Belardi, Francesco Grasso, Antonio Luchetta, Stefano Manetti, Maria Cristina Piccirilli, "Neural Network-Based Fault Diagnosis of Joints in High Voltage Electrical Lines", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 4, pp. 488–498, 2020. doi: 10.25046/aj050458
- Putta Nageswara Rao, Suresh Babu Valer, Koka Naga Sai Suman, "Improvement of Desirable Thermophysical Properties of Soybean Oil for Metal Cutting Applications as a Cutting Fluid", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 3, pp. 129–134, 2020. doi: 10.25046/aj050317
- Ilyas Lahlouh, Ahmed Elakkary, Nacer Sefiani, "Design and Implementation of State-PID Feedback Controller for Poultry House System: Application for Winter Climate", Advances in Science, Technology and Engineering Systems Journal, vol. 5, no. 1, pp. 135–141, 2020. doi: 10.25046/aj050118
- Paolo Mercorelli, "Stability Analysis of a Linear Model Predictive Control and its Application in a Water Recovery Process", Advances in Science, Technology and Engineering Systems Journal, vol. 4, no. 5, pp. 314–320, 2019. doi: 10.25046/aj040540
- Waweru Njeri, Minoru Sasaki, Kojiro Matsushita, "Two Degree-of-Freedom Vibration Control of a 3D, 2 Link Flexible Manipulator", Advances in Science, Technology and Engineering Systems Journal, vol. 3, no. 6, pp. 412–424, 2018. doi: 10.25046/aj030649
- Kanasottu Anil Naik, Chandra Prakash Gupta, Eugene Fernandez, "Performance improvement of a wind energy system using fuzzy logic based pitch angle control", Advances in Science, Technology and Engineering Systems Journal, vol. 3, no. 3, pp. 30–37, 2018. doi: 10.25046/aj030304
- Hajer Jmii, Asma Meddeb, Souad Chebbi, "Enhancement of Voltage Stability through Optimal Location of UPFC", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 6, pp. 261–266, 2017. doi: 10.25046/aj020631
- Hajer Jmii, Asma Meddeb, Souad Chebbi, "Heuristic Based Approach for Voltage Stability Improvement using FACTS Devices", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 6, pp. 252–260, 2017. doi: 10.25046/aj020630
- Fatima Al-Mutairi, Reem Al-Mutairi, "Stability and nonlinear controller design of Fast-Lock Phase-Locked Loop in 0.18-µm CMOS", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 3, pp. 1266–1271, 2017. doi: 10.25046/aj0203160
- Mohammed Omar Benaissa, Samir Hadjeri, Sid Ahmed Zidi, "Impact of PSS and SVC on the Power System Transient Stability", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 3, pp. 562–568, 2017. doi: 10.25046/aj020372
- Fatma Abdelhedi, Nabil Derbel, "A delay-dependent distributed SMC for stabilization of a networked robotic system exposed to external disturbances", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 3, pp. 513–519, 2017. doi: 10.25046/aj020366
- Marwa Fathallah, Fatma Abdelhedi, Nabil Derbel, "A synchronizing second order sliding mode control applied to decentralized time delayed multi-agent robotic systems: Stability Proof", Advances in Science, Technology and Engineering Systems Journal, vol. 2, no. 3, pp. 160–170, 2017. doi: 10.25046/aj020321
- Tariq Kamal, Syed Zulqadar Hassan, "Energy Management and Simulation of Photovoltaic/Hydrogen /Battery Hybrid Power System", Advances in Science, Technology and Engineering Systems Journal, vol. 1, no. 2, pp. 11–18, 2016. doi: 10.25046/aj010203
- Niaz Ahmed, Kashif Ahmed, "Chemical and Different Nutritional Characteristics of Brown Seaweed Lipids", Advances in Science, Technology and Engineering Systems Journal, vol. 1, no. 1, pp. 23–25, 2016. doi: 10.25046/aj010104
